.jpeg)
Organic chemistry is the branch of chemistry that studies the structure, properties, and reactions of organic compounds.
One of the most important reactions in organic chemistry is the Wurtz reaction, which is used to synthesize alkyl chains.
In this article, we will discuss what the Wurtz reaction is, how it works, and its applications.
What is the Wurtz Reaction?
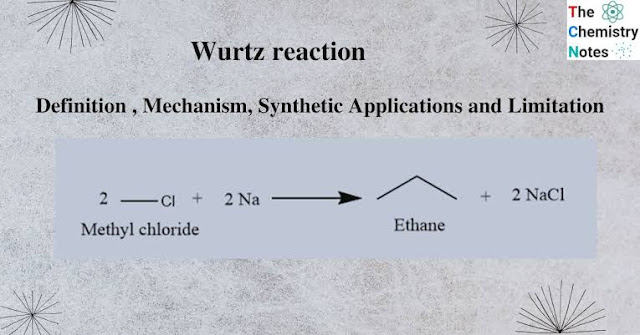
The Wurtz reaction is a chemical reaction in which two alkyl halides are reacted with metallic sodium in dry ether to produce a higher alkane.
The reaction is named after the German chemist Charles Adolphe Wurtz who first described the reaction in 1855. The general equation for the Wurtz reaction is as follows:
2 R-X + 2 Na → R-R + 2 NaX
Where R is an alkyl group and X is a halogen, usually chlorine or bromine.
The reaction proceeds through a free radical mechanism, which involves the formation of an intermediate species known as an alkyl radical.
Mechanism of the Wurtz Reaction:
The Wurtz reaction proceeds through a free radical mechanism. The reaction can be broken down into three main steps:
Step 1: Formation of the alkyl radical
The first step in the Wurtz reaction is the formation of an alkyl radical. This is achieved by the reaction of an alkyl halide with metallic sodium.
The metallic sodium donates an electron to the halogen, which causes the halogen to leave as a halide ion, leaving behind an alkyl radical.
Step 2: Formation of the dimer
The alkyl radical then reacts with another alkyl radical to form a dimer. This step is also known as the coupling step.
Step 3: Formation of the final product
The final step in the Wurtz reaction is the elimination of sodium halide, which results in the formation of the final product, which is a higher alkane.
Applications of the Wurtz Reaction:
The Wurtz reaction has many applications in organic synthesis. Some of the most important applications are discussed below.
Synthesis of Alkanes:
The Wurtz reaction is used extensively in the synthesis of alkanes. The reaction is particularly useful for the synthesis of long-chain alkanes, which are difficult to obtain using other synthetic methods.
Synthesis of Biphenyls:
The Wurtz reaction can also be used to synthesize biphenyls. Biphenyls are a class of organic compounds that are used as intermediates in the synthesis of many important organic compounds, including pharmaceuticals and polymers.
Synthesis of Higher Alkyl Substituted Aromatics:
The Wurtz reaction can also be used to synthesize higher alkyl substituted aromatics. These compounds are important intermediates in the synthesis of many organic compounds, including pharmaceuticals and agrochemicals.
Limitations of the Wurtz Reaction:
Despite its many applications, the Wurtz reaction has some limitations. One of the main limitations is that the reaction only works with primary and secondary alkyl halides.
Tertiary alkyl halides do not undergo the Wurtz reaction because they are too sterically hindered. Additionally, the reaction is not suitable for the synthesis of highly branched alkanes.
Conclusion:
The Wurtz reaction is a highly useful reaction in organic synthesis. The reaction is used extensively for the synthesis of alkanes, biphenyls, and higher alkyl substituted aromatics. While the reaction has some limitations, it remains an important tool for organic chemists.
Comments
Post a Comment