 |
| how did Bohr Explain the Line Spectrum of Hydrogen |
Question: how did bohr explain the line spectrum of hydrogen?
Answer: Bohr explained the line spectrum of hydrogen by proposing that electrons orbit the nucleus in distinct energy levels. When electrons transition between these levels, they emit or absorb energy in the form of discrete packets called photons. The emitted photons correspond to specific wavelengths, creating the observed line spectrum. Bohr's model introduced the concept of quantized energy levels and formed the basis for modern quantum theory.
Introduction:
Bohr's explanation of the line spectrum of hydrogen revolutionized our understanding of atomic structure and laid the foundation for modern quantum theory.
In this article, we will delve into the details of how Niels Bohr's groundbreaking model clarified the mysterious nature of hydrogen's line spectrum.
Let's explore the key concepts and principles behind Bohr's explanation and its significance in the field of physics.
Section 1: The Riddle of the Hydrogen Line Spectrum
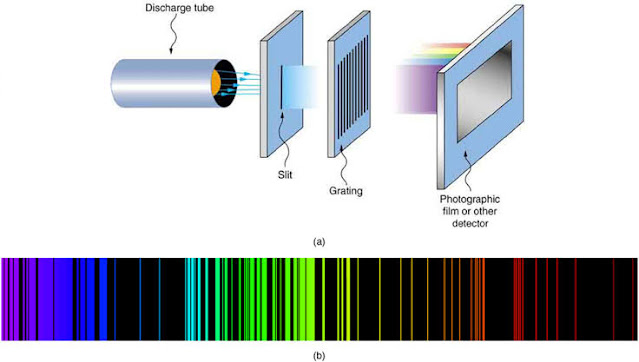
The line spectrum of hydrogen, discovered by scientists in the late 19th century, presented a perplexing challenge. When hydrogen gas was excited with an electric current, it emitted light at specific wavelengths, producing a series of distinct colored lines. The question was: What caused this unique pattern, and how could it be explained?
Section 2: Bohr's Model: Electrons in Fixed Energy Levels
Niels Bohr proposed a revolutionary model in 1913, suggesting that electrons orbit the nucleus of an atom in distinct energy levels, or shells. According to Bohr, electrons occupy stable orbits around the nucleus without losing energy. Each energy level corresponds to a specific fixed energy value.
Section 3: Quantized Energy and Emission of Photons
Bohr further postulated that electrons can transition between energy levels by absorbing or emitting energy in discrete packets called photons. When an electron moves from a higher energy level to a lower one, it releases a photon of a specific wavelength corresponding to the energy difference between the two levels.
Section 4: The Balmer Series and the Hydrogen Line Spectrum
Bohr's model successfully explained the hydrogen line spectrum by applying his principles of quantized energy levels. The most prominent series observed in hydrogen's spectrum is the Balmer series, which consists of visible light wavelengths. The Balmer series can be mathematically described using the following equation:
1/λ = R_H[(1/n_final^2) - (1/n_initial^2)]
Here, λ represents the wavelength of the emitted light, R_H is the Rydberg constant, and n_final and n_initial are the energy levels of the electron.
Section 5: Implications and Significance
Bohr's explanation of the line spectrum of hydrogen provided a critical step forward in our understanding of atomic structure and quantum mechanics. It introduced the concept of discrete energy levels and the quantization of energy in atoms, challenging classical physics. Bohr's model formed the basis for further developments in quantum theory and contributed to our understanding of atomic spectra beyond hydrogen.
Conclusion:
Niels Bohr's explanation of the line spectrum of hydrogen using his revolutionary atomic model paved the way for modern quantum theory. By introducing the concept of quantized energy levels and the emission of photons, Bohr provided a coherent framework for understanding the mysterious patterns observed in hydrogen's line spectrum. This groundbreaking work not only resolved a long-standing scientific puzzle but also laid the foundation for further advancements in atomic physics.
Comments
Post a Comment